link
Name Reactions
Please use the following URL if you want to set a link: www.organic-chemistry.org/namedreactions/ .
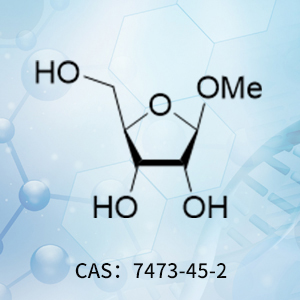
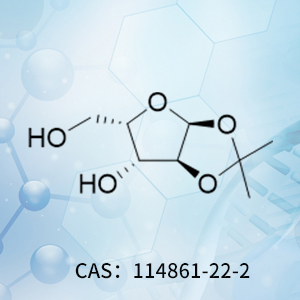
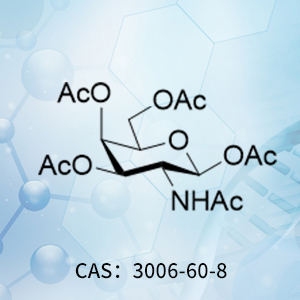
Product of the Month
Acetoacetic Ester Condensation
Azide-Alkyne 1,3-Dipolar Cycloaddition
Baker-Venkataraman Rearrangement
Barton-McCombie Reaction (Barton Desoxygenation)
Bohlmann-Rahtz Pyridine Synthesis
Buchwald-Hartwig Cross Coupling Reaction
Cannizzaro Oxidation Reduction
Corey-Bakshi-Shibata Reduction
Criegee Mechanism for Ozonolysis
Curtius Rearrangement (Reaction)
Hantzsch Dihydropyridine Synthesis (Pyridine Synthesis)
Hell-Volhard-Zelinsky Reaction
Horner-Wadsworth-Emmons Reaction
Johnson-Corey-Chaykovsky Reaction
Kulinkovich-de Meijere Reaction
Kulinkovich-Szymoniak Reaction
Meerwein-Ponndorf-Verley Reduction
Myers' Modification of the Ramberg-Bäcklund Reaction
Nucleophilic Substitution (SN1 / SN2)
Paal-Knorr Thiophene Synthesis
Ring Opening Metathesis (Polymerization)
Van Leusen Imidazole Synthesis
- Previous:Named Reactions in Organic Chemistry
- Next:Organic Chemistry